WATER SOFTENER WORKING PROCESES
Summary for: Water softening
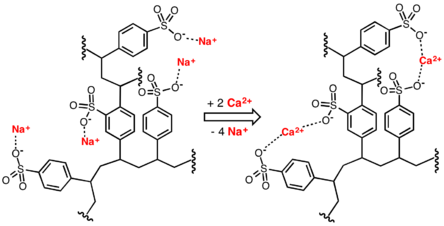
In this application, ion-exchange resins are used to replace the magnesium and calcium ions found in hard water with sodium ions. When the resin is fresh, it contains sodium ions at its active sites. When in contact with a solution containing magnesium and calcium ions (but a low concentration of sodium ions), the magnesium and calcium ions preferentially migrate out of solution to the active sites on the resin, being replaced in solution by sodium ions. This process reaches equilibrium with a much lower concentration of magnesium and calcium ions in solution than was started with.
Idealised image of water-softening process, involving replacement of calcium ions in water with sodium ions donated by a cation-exchange resin
The resin can be recharged by washing it with a solution containing a high concentration of sodium ions (e.g. it has large amounts of common salt (NaCl) dissolved in it). The calcium and magnesium ions migrate from the resin, being replaced by sodium ions from the solution until a new equilibrium is reached. The salt is used to recharge an ion-exchange resin, which itself is used to soften the water.
Cation-exchange resin
Cation exchange method removes the hardness of water but induces acidity in it. Which is further removed in next stage of treatment of water by passing this acidic water through anion exchange process Formula: R−H acidic
Reaction: R−H + M+ = R-M+ + H+.